Wearable tech startup pursuing regulatory approval in the US and Europe for the treatment of depression, anxiety and insomnia.
InvestAddressing the Mental Health Crisis
85 million US adults experience symptoms of depression or anxiety, and 70 million suffer from sleep deprivation. These problems have similar scale in Europe. Drug therapy and behavioral therapy do not sufficiently or affordably address the growing mental health crisis.

Proven Product-Market Fit
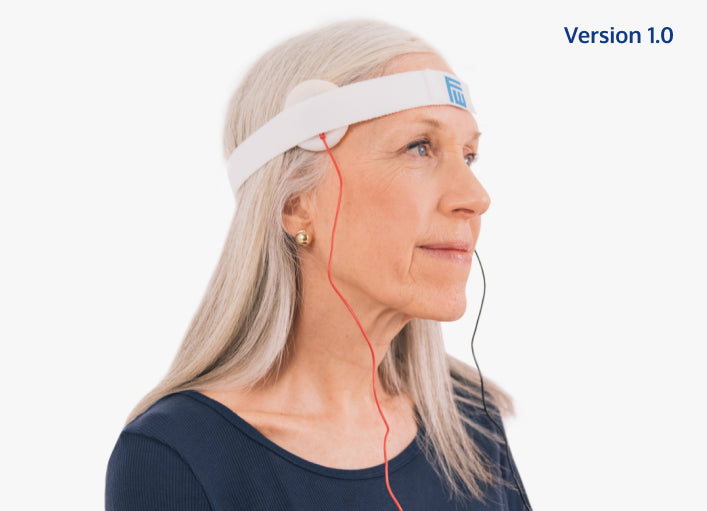
Our Version 1.0
Under temporary FDA-Clearance, Fisher Wallace distributed 100,000 Version 1.0 devices via 14,000 prescribers to treat depression, anxiety and insomnia, and was named one of "5 Health-Tech Startups to Watch" by Entrepreneur magazine.
Introducing OAK

Rewiring The Standard of Care
The company is nearing completion of its patent-pending Version 2.0 device, OAK, while pursuing FDA approval and CE Marks (Europe/UK) for the treatment of depression, anxiety and insomnia. FDA temporary clearance expiring, see details below*
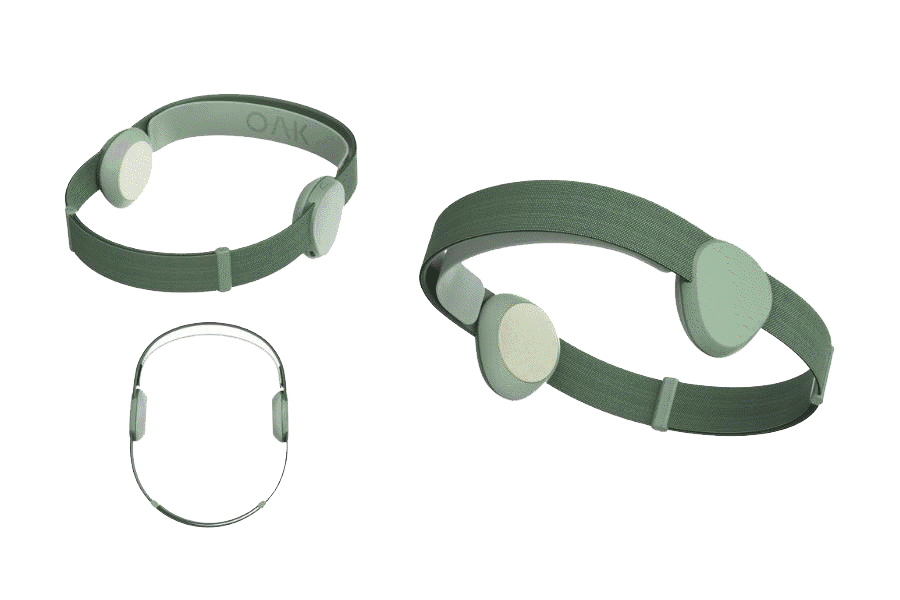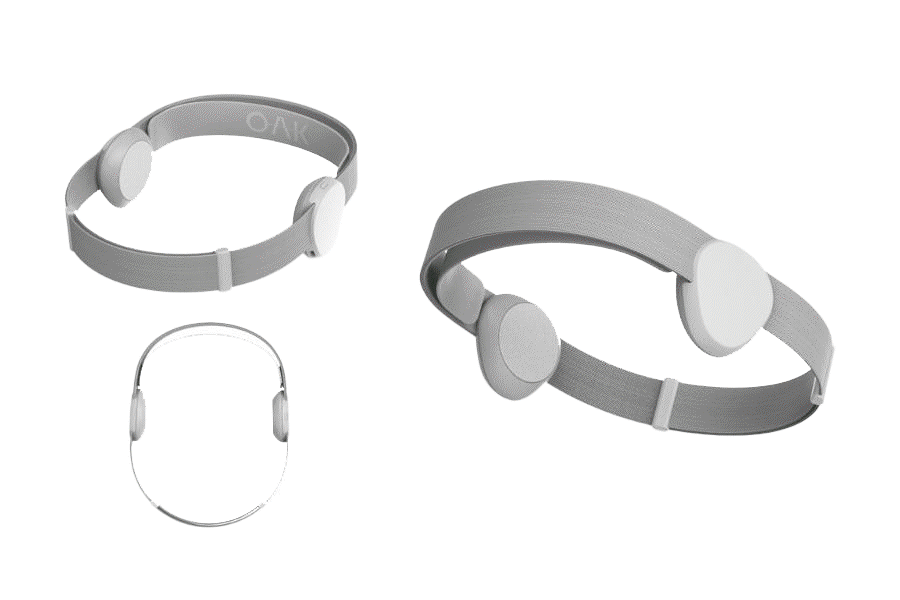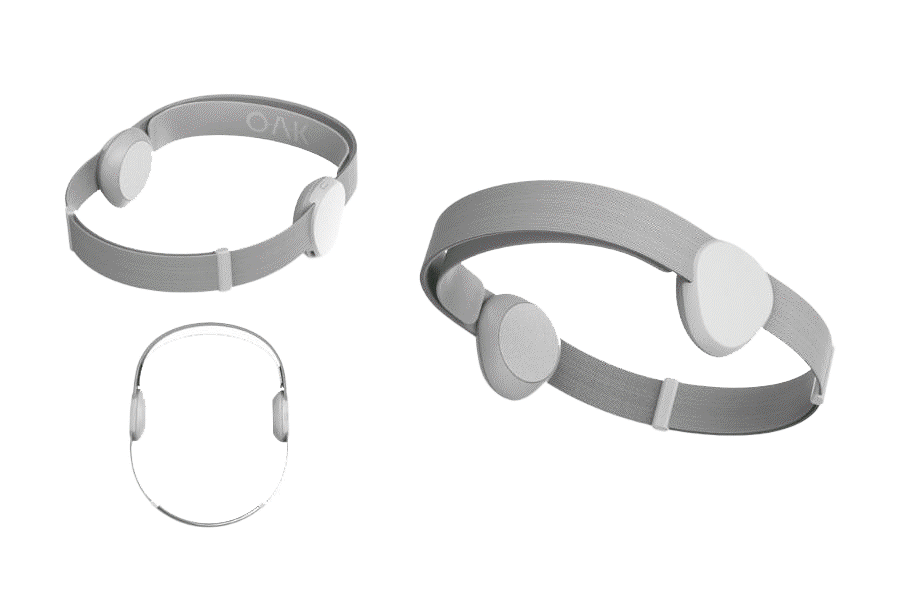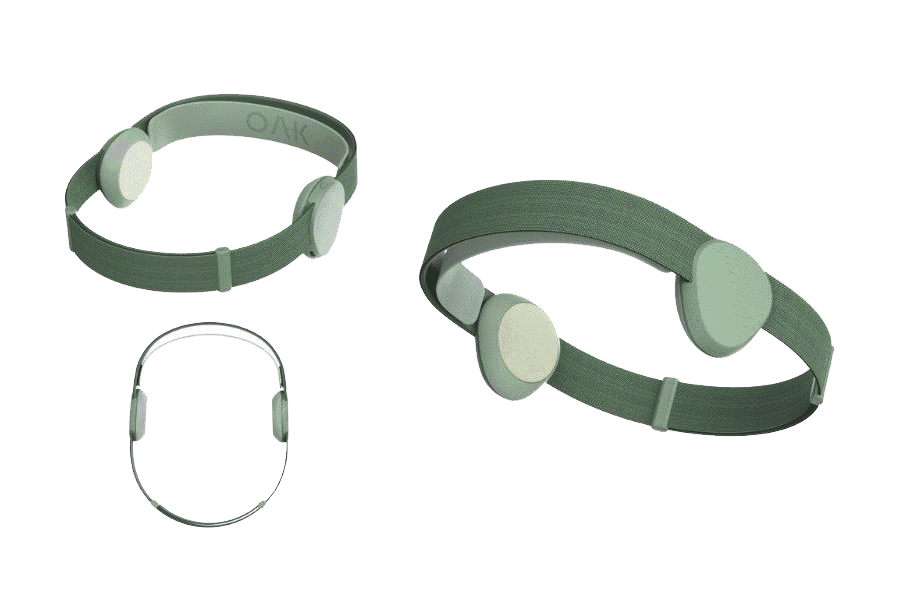
Groundbreaking Neuroscience
Fisher Wallace recently completed a 255-subject, triple-blind, randomized, controlled trial for the acute treatment of major depressive disorder (pending publication), and is sponsoring an ongoing anxiety and insomnia study in collaboration with the Seattle Police Department.
OAK is designed to appeal to millions of people in need of better mental health.
InvestRapid Treatment Without Serious Side Effects
The company's recent depression study demonstrated rapid-action without causing serious side effects. Additional research conducted over the next 12 months is intended to obtain approval in the US and Europe/UK. FDA temporary clearance expiring, see details below*


Kelly Roman
CO-FOUNDER, CEO, DIRECTOR
